WuXi Biologics
Offering End-to-End Solutions
案例分析
研发服务的范例分析
范例分析: 成药性研究
背景
某客户要求药明生物对他们提供的两个候选分子的关键表征做评估以决定一个可用作后续CMC开发的最终分子。
挑战
需要开发出一个成药性研究的平台,重点是需要遴选出能够决定成药性的关键筛选标准和关键产品表征,以挑出最终的分子和克隆。
解决方案/结果
- 两个候选分子所构建出的细胞群产量相同。
- 然而分子1的产物有聚体形成,且溶解度比较低,纯化方面也有问题。
- 根据表1里所列出的多个因素,我们做了一个综合评价,决定选择分子2来继续完成细胞株的克隆和后续的CMC开发工作。
- 在后续开发和优化后,分子2所构建的克隆最终达到了9.7g/L的产量,并于2016年4月完成新药临床申报。
| 标准 | 分子1 | 分子2 |
|---|---|---|
| 是否适合上游开发 | 3.7 g/L | 3.7 g/L |
|
是否适合下游开发 |
在ProA纯化后有大于10%的高聚物; 阳离子交换层析的收率为52% |
阳离子交换层析的收率为87% |
| 制剂稳定性 | 平台方法得到的溶解度:100mg/ml | 平台方法得到的溶解度:>150mg/ml |
| 生物物理和生物化学的表征 | DSC: Tm2: 76.4oC | DSC: Tm2: 78.7oC |
范例分析: 如何在表达量和产品质量之间寻求平衡
背景
客户要求构建一个产量大于1.5g/L并且质量优异的细胞株。同时他们还要求被选出的克隆细胞株一定要保证单克隆性。
挑战
如何在非常紧凑的时间表内满足客户的要求。
解决方案/结果
- 在转染前先对该分子的DNA序列进行了优化,并在细胞株构建中采用了双筛选的系统。同时我们还用ClonePix对该细胞系进行了两轮克隆以保证单克隆性。
- 进一步评价了两种不同的培养基和批次补料的培养条件,得到了大幅超出客户预期的产量。(见表1的产量结果)
- 最终在客户要求的时间内按时交付合格的细胞株。
| 标准 | 结果 |
|---|---|
| 产量 |
细胞群产量:1.5 g/L 克隆产量:3.6 g/L |
|
单克隆率 |
保存并记录好铺板前细胞在ViCell拍出的图片以及用ClonePix挑出的克隆照片作为单克隆的证明 |
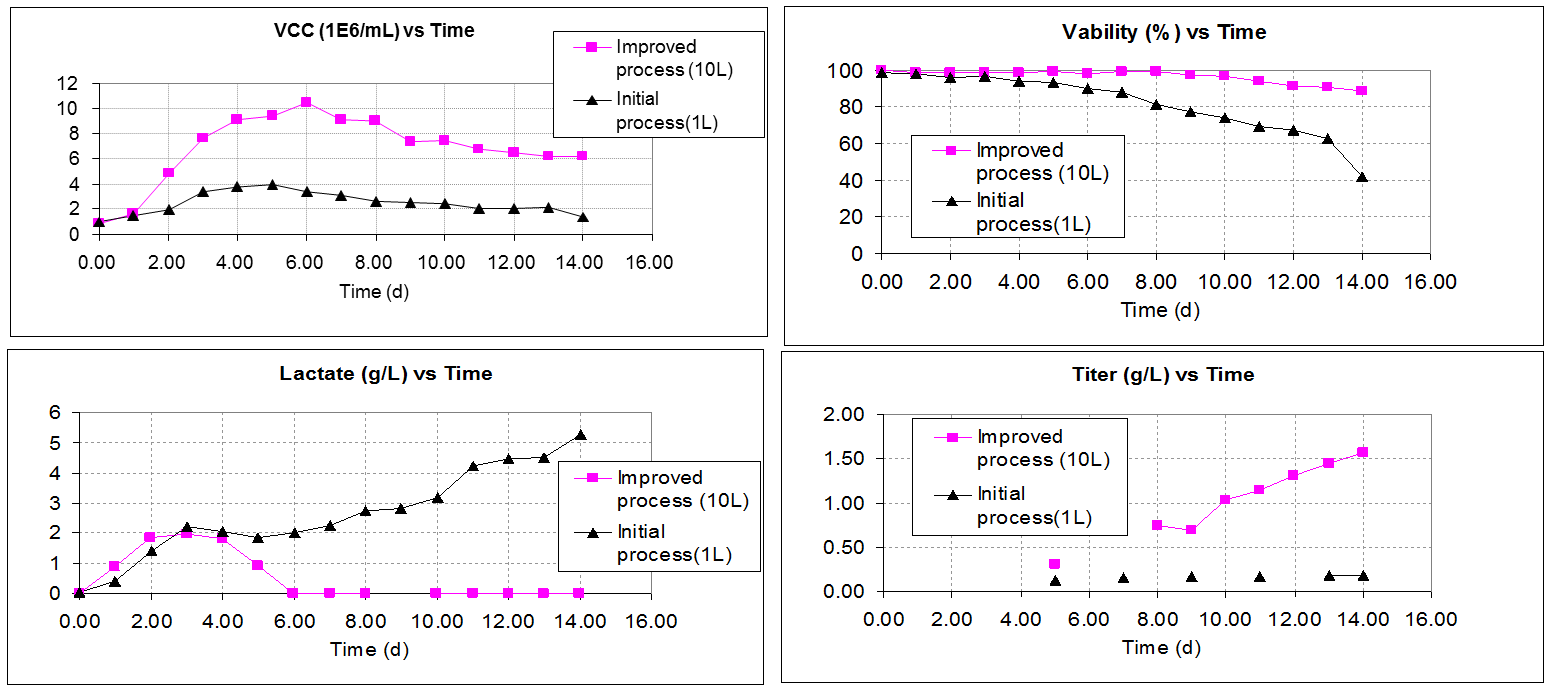
范例分析:对剪切力敏感性克隆的策略调整
背景
- 初始工艺(原工艺)的细胞生长水平低,乳酸积累多,最终蛋白产量很低只有0.2g/L。
挑战
- 克隆具有剪切力敏感性,在使用微泡底部通气条件下生长状况很差。
解决方案/结果
- 药明生物团队使用大孔气体分布器来替代微泡分布器并优化反应器培养条件。
- 我们通过优化基础培养基和流加/补料培养基来进一步改善工艺。
- 以上所做的努力使得细胞生长状态,细胞活率,乳酸变化水平均达到更优状态,并且最终蛋白产量提高到>5g/L。
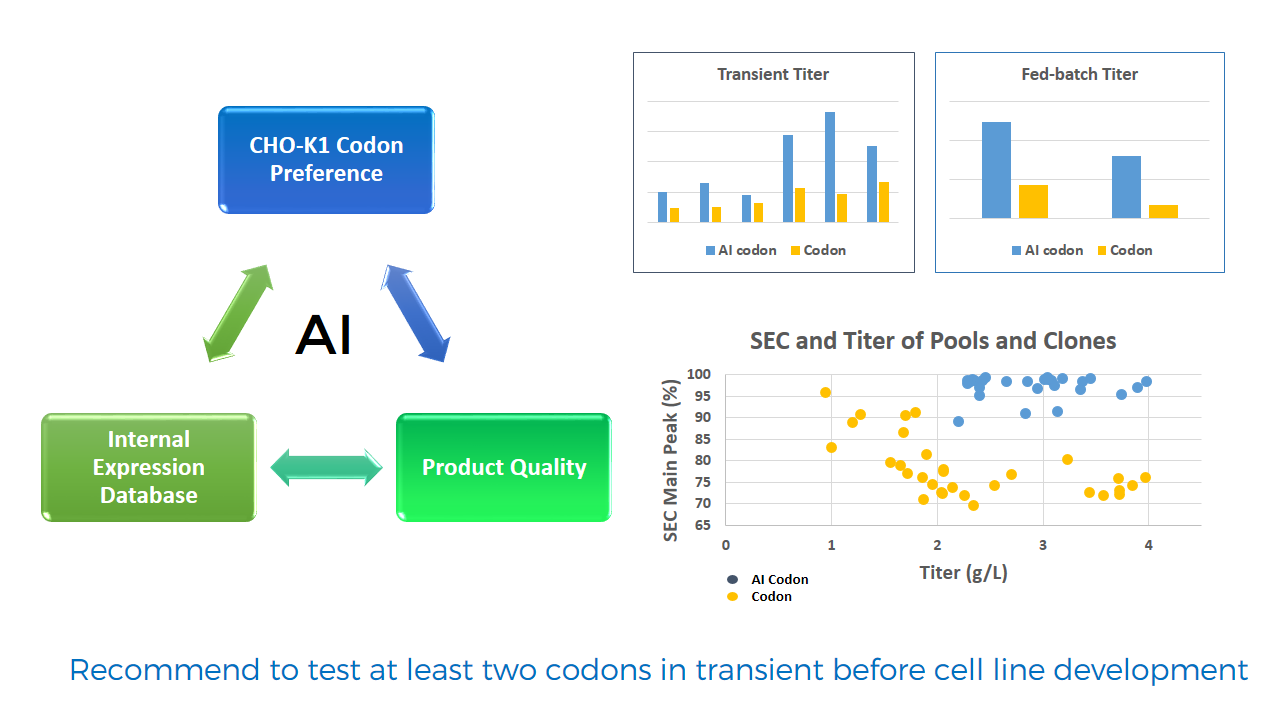
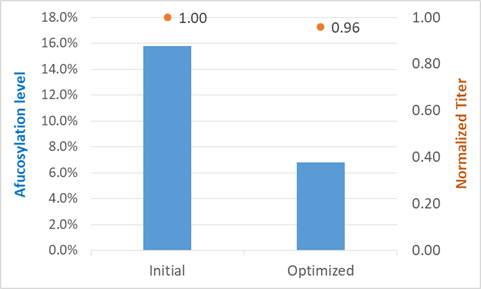
范例分析:优化糖型分布以降低G0F含量
背景
- 当与对照样品比较时,起始工艺具有很大差异的糖型分布尤其对于G0F。
挑战
- G0F含量大于15%的情况高于对照样品。
- 对蛋白产物的影响必须是可控制的,同时做出工艺改变。
解决方案/结果
- 加入培养基组分X可成功降低G0F含量。
- 组分X会使蛋白产量下降但是可以降低G0F含量。
- 改善后的工艺具有令人满意的糖型分布和可接受范围的蛋白产量。
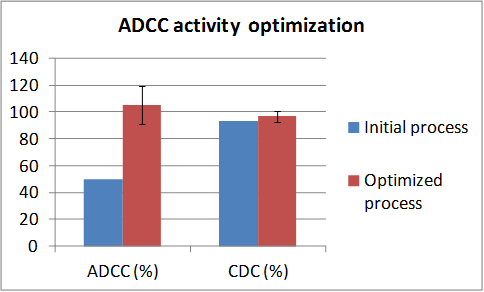
范例分析:抗体依赖的细胞介导的细胞毒作用(ADCC)途径的优化
背景
- 与对照样品比较时,原始工艺对应的ADCC活性大大降低。
挑战
- 明显降低的ADCC活性(大约低50%)很难进行优化。
- 补体依赖的细胞毒作用(CDC)活性需要维持在一定范围而只是优化ADCC活性。
解决方案/结果
- 加入培养基组分使得ADCC活性调节并且对CDC活性没有明显影响,优化后使ADCC和CDC活性都在靶向范围内。
- 在ADCC活性与非岩藻糖基化含量总和(G0+G1+G2+Man5)之间强烈的相关性。
- 对于需要ADCC功能的分子来说这是一个宝贵的工具。
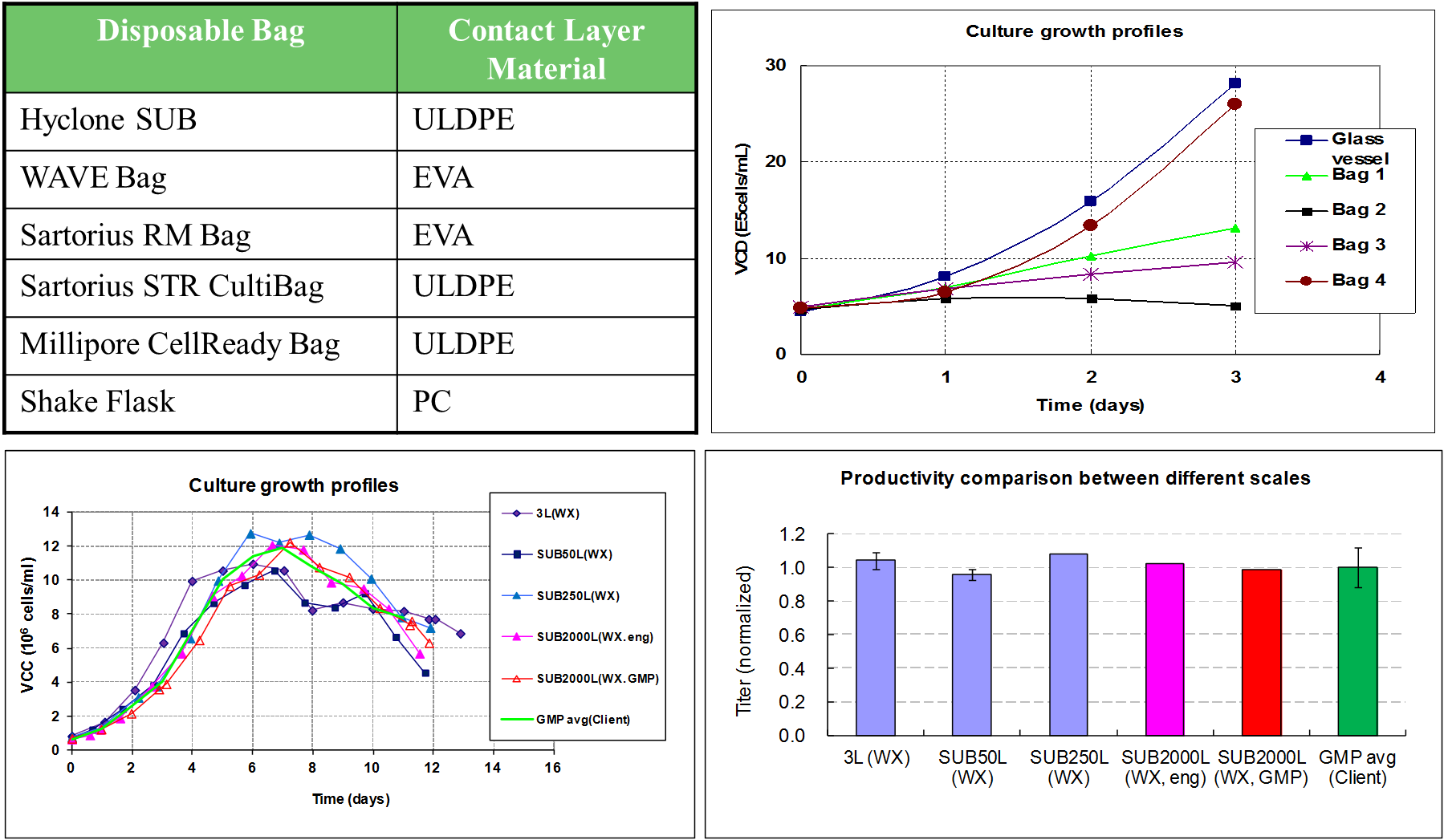
范例分析:用一次性生物反应器培养NS0细胞
背景
- 由客户将工艺直接转移到药明生物进行临床试验生产。
- 客户对平台生产工艺和最终产品要求做可比性评估。
挑战
- 原始工艺的开发者已不在该部门,只能进行基于文件方面的工艺技术转移。
- 新工艺用一次性反应器来运行,然而原始工艺是通过固定不锈钢材质的反应器(包括管路系统)来完成。
- 根据历史数据来看,NS0细胞系很难在一次性反应器系统中进行培养。
解决方案/结果
- 通过工艺开发团队的巨大努力,生产工艺已被成功的转移,优化并且在一次性反应器系统中运行。
- 一次性系统中工艺的放大在50L和250L规模的反应器中得到确认,类似地GMP生产也在2000L规模的一次性生物反应器中进行。
- 在研究中的新药(IND)申报中可比性数据已被美国食品及药物管理局(FDA)所接受。
- 对于全球临床试验,在中国生产的第一个生物药已在位于无锡市的药明生物生产基地成功完成生产。
范例分析:通过DOE实验来优化改进CEX方法
背景
- 原始工艺满足蛋白目标产量,基础培养基和补料培养基已经锁定。然而,将酸性异构体从大约28%降低至到15%,通过这种方法客户想要进一步改善产品质量。
挑战
- 工艺培养基已经锁定,只能在工艺条件上进行优化。
解决方案/结果
- 执行DOE实验研究,其中温度和pH是主要输入变量(两个已知的主要工艺条件会影响电荷异构体)。
- 获得一个设计空间,在此范围内蛋白产量可以保持在一定水平而且酸性异构体水平可降低至13%。
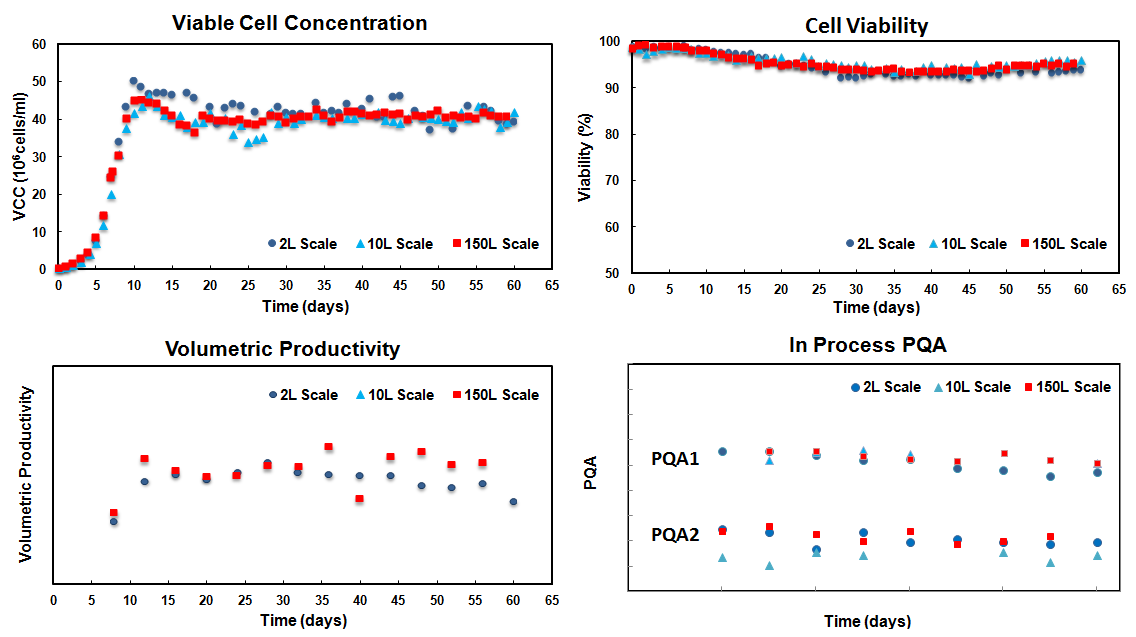
范例分析:实现高细胞密度和细胞活率的稳态灌流培养
背景
- 对产量和产品质量有较高要求的重组蛋白。
挑战
- 相对不稳定的产品。
- 产品质量属性(PQA)对细胞培养的表现非常敏感比如细胞活率。
解决方案/结果
- 由于蛋白的自然属性,比产量因而受到限制。要求活细胞密度在高水平从而获得更大的蛋白产量。
- 引进交替切向流细胞截留系统(ATF)作为细胞滞留器来获得极高的活细胞密度;适当的细胞移除速率(从截流系统中流出)被用来维持稳定的活细胞密度和细胞活率。
- 长周期的稳态灌流(通常为60天左右,最多可达130天)可实现稳定的细胞生长状态、代谢水平、最终产量和产品质量属性
- 从ATF最终收获的澄清培养液使得产物直接(未经处理)进入下游工艺成为可能。
- 灌流工艺已经成功地放大到250L规模(其中工作体积为150L)的GMP生产运行。
Case Study: FTE-Model Approach
背景
WuXi Biologics suggested using an FTE approach to meet critical bispecific antibody development timelines.
挑战
Previous client attempts to produce and purify the protein led to undesirable expression levels of a bispecific antibody due to low expression of one of the antibody chains which resulted in only 10% of desired protein product from the entire protein production campaign. These challenges were delaying product CMC development and thus time was critical as original IND filing date was in jeopardy.
解决方案/结果
After discussion with the client and outlining the DOE study required to potentially solve the problem, client-dedicated personnel via an FTE program was established. The client-dedicated FTE team generated and evaluated 42 different constructs and evaluated expression levels of the bispecific antibody in over 1,000 minipools using a high-throughput methodology. Minipools demonstrating best results were moved to clone screening and eventual final clone selection. Results provided in Table 3.
Table 3 – FTE program results| Final Titer of bispecific Ab clone | Main product expression levels before purification | Main product purity after purification | Date of client IND filing |
|---|---|---|---|
| 3.8 g/L | 90% | 98% | (On-time filing) |
Case Study: Protein Refolding Analysis using HT-Approach
背景
Client struggled with developing the tools to understand if a recombinant cytokine product produced in e. coli had refolded correctly after removal from inclusion bodies post fermentation.
挑战
Develop a high-throughput system that could quickly evaluate over 130 different molecules using multiple analytical methods to meet critical client timelines and budget.
解决方案/结果
A well-established, HT matrix-screening method in a fast, efficient way.

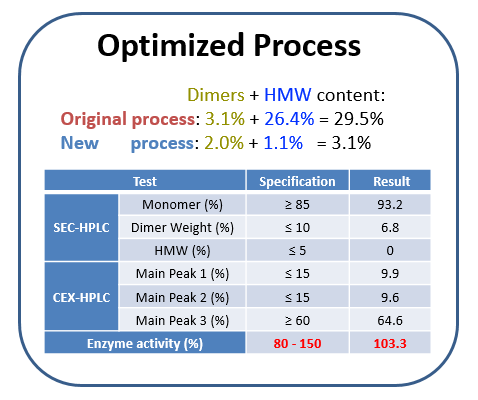
Case Study: Trouble-shooting product issues in GMP manufacturing
背景
Client could not get a consistent product quality or GMP manufacturing process performance at current vendor. Requested WuXi Biologics assemble a team from multiple functional areas to trouble-shoot manufacturing process and provide solutions.
挑战
Product had high HMW and dimer content and low enzymatic activity. Had to evaluate over 800 pages of batch records, perform technical transfer and trouble-shoot based on investigation master plan in short time frame.
解决方案/结果
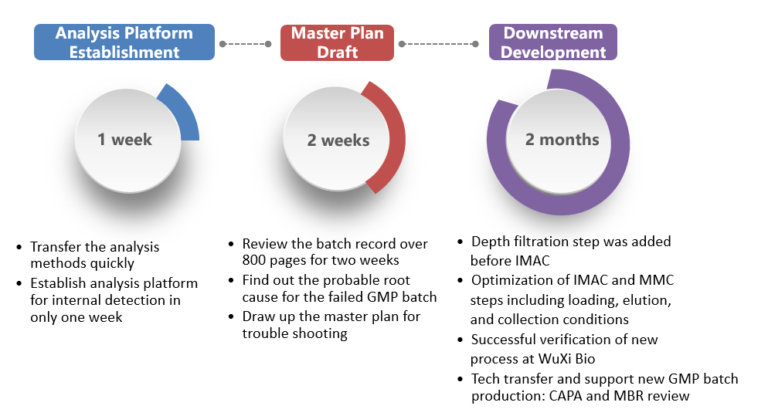










 点击此链接看大图
点击此链接看大图