药明生物
提供端到端一站式解决方案
概述
全球领先的开放式、一体化生物偶联制药能力和技术赋能平台

Watch our video to learn more about the WuXi XDC integrated services advantage
药明合联(WuXi XDC)是由药明生物和合全药业联合成立的合资公司,是全球领先的开放式、一体化生物偶联制药能力和技术赋能平台。公司为全球生物偶联制药公司和生物偶联药开发者们提供全方位的端到端CRDMO服务,实现从概念到商业化生产的全过程整合,并通过高度集成ADC工艺研发和生产的供应链,加速并变革全球生物偶联药研发进程,为客户提供开放式,一体化的偶联制药技术平台,降低研发成本,造福病患。
药明合联已成功赋能多个ADC药物在15个月以内完成从DNA到新药临床试验申请(IND),相比传统开发时间几乎缩短了一半。截止2021年12月底,有60个处于CMC阶段的ADC及其他偶联药物在药明合联平台上开发,其中包括22个已提交IND的项目,6个项目进入后期临床试验阶段。
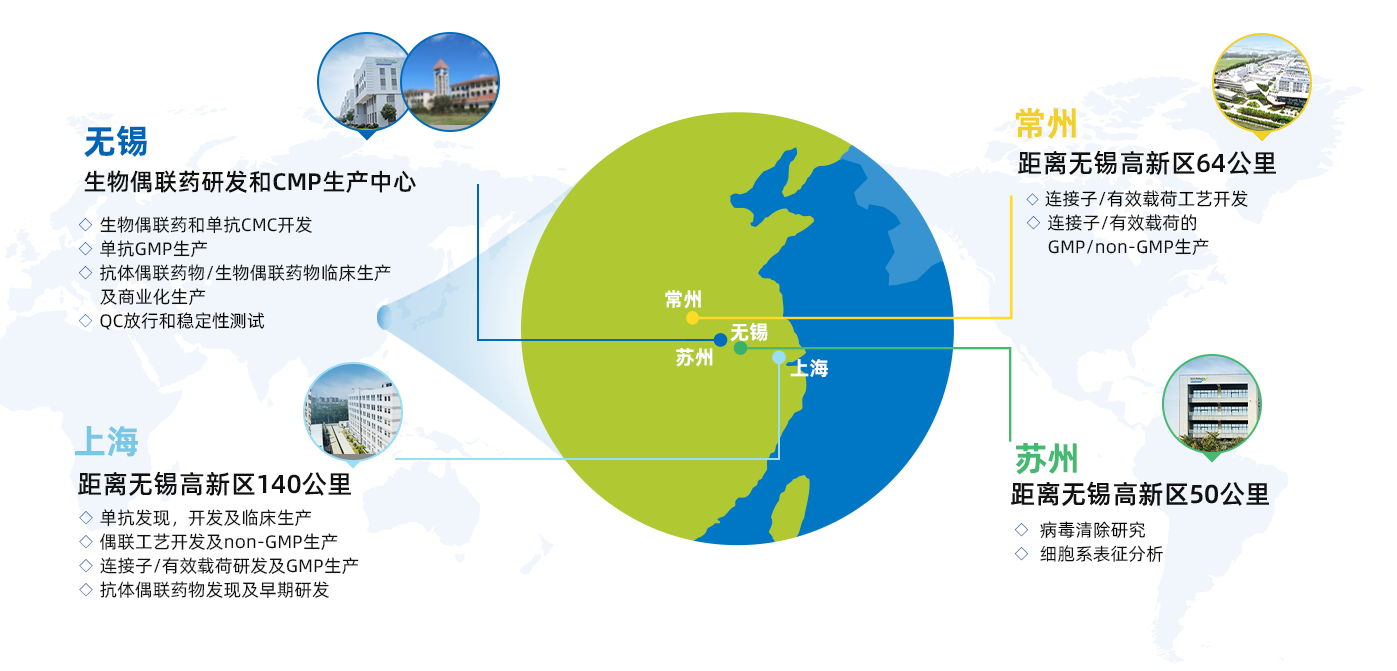
药明合联技术平台内的所有活动均在1-2小时车程范围内区域进行,从而提供了前所未有的以地理为中心的供应链和价值链。
药明合联主要由三个业务部门组成,上海生物偶联研发部负责偶联药物的早期研究和工艺开发,无锡高新区的生物偶联药园区负责偶联药物的GMP偶联生产,成品制剂及工艺开发和制剂生产,常州小分子基地承担载荷和连接子的研发和生产。
更多参考信息
白皮书: The Watershed Moment for ADCs has Arrived
行业观点:
Antibody Drug Conjugate (ADC) Development And Manufacturing Challenges And Solutions
Webinar:
Overcoming Antibody-Drug Conjugate (ADC) Process Development
















